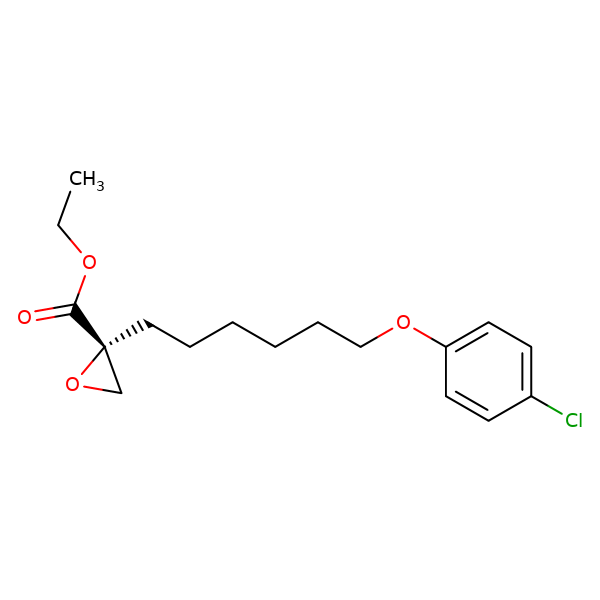
Etomoxir, or rac-Ethyl 2-[6-(4-chlorophenoxy)hexyl]oxirane-2-carboxylate, in form of the dextrorotatory (R)-( )- enantiomer, is an irreversible inhibitor of carnitine palmitoyltransferase-1 (CPT-1; EC 2.3.1.21) on the inner face of the outer mitochondrial membrane. The actual inhibitor – (R)-( )-etomoxir-Coenzym A ester – is formed in an intracellular process. The middle inhibitor concentration for the inhibition of the CPT-1 in the liver, heart, and muscle mitochondria of rats lies in between 5 and 20 nmol/l (for rac-Etomoxir), depending on the animal's state of metabolism (fed or fasting). ( )-Etomoxir is a colourness solid with a melting point of 38 °C (311 K). The sodium salt of ( )-Etomoxir is water-soluble. The (S)-(-)-enantiomer of Etomoxir does not block CPT-1.
Etomoxir's mechanism prevents the formation of acyl carnitines, a step that is necessary for the transport of fatty acyl chains from the cytosol into the intermembrane space of the mitochondria. This step is essential to the production of ATP from fatty acid oxidation. Etomoxir has also been identified as a direct agonist of PPARα. An off-target effect has been demonstrated at high concentrations of Etomoxir on Coenzyme-A (CoA) metabolism, and on complex I of the electron transport chain.
The influence of Etomoxir on food uptake is a matter of discussion. Contradictory findings were reported.
Clinical development
The primary effect of Etomoxir in vivo is a decrease in ketone bodies in the blood, followed by a decrease in blood glucose levels. These pharmacodynamic effects of ( )-Etomoxir can be explained by its mechanism and as a consequence of the inhibition of long-chain fatty acid oxidation. This results in a depression of ketogenesis and gluconeogenesis in the liver, and via disinhibition of the pyruvate dehydrogenase in an activation of glucose oxidation in the muscle. In 1980, this prompted German firm Byk Gulden Lomberg Chemische Fabrik GmbH – the patent owner – to initiate drug development for the treatment of type 2 diabetes. Because of the insufficient anti-diabetic efficacy and due to the fact that in the toxicological trials a heart hypertrophy in rats was found, Byk Gulden decided to cease development in 1992, before entering phase III clinical research. The company had found by then a mild anti-diabetic effect and a good safety profile with exception of few cases of transient increases of liver transaminase (GPT). The most promising effect found was the lowering of triglyceride levels in blood.
The results of the first clinical trial with Etomoxir in patients with chronic congestive heart failure were published in 2000. Throughout the following years, it was found that Etomoxir has beneficial effects either in isolated perfused rat hearts or in vivo in animals and humans.
By 1999, the inventor had granted a license to MediGene AG (Martinsried, Germany) for further development as a drug against congestive heart failure and hyperlipidemia. Phase II clinical research started 2001, and in 2002, Medigene AG announced that it had terminated this trial due to adverse side effects, i.e., unacceptable high liver transaminase levels in 4 patents in the treatment group. The 2007 publication of the statistical evaluation, however, indicates that there were no significant differences between the placebo and treatment groups.
Further developments
Throughout the late 2000s, and 2010s, experimental evidence has accumulated indicating a broad spectrum of biological effects of Etomoxir. Among the reported effects are reduced diseas progression in multiple sclerosis, resistance to onset of Parkinson's disease, and a reversal of autoimmune encephalitis. In addition, etomoxir inhibits tumor growth in animal models of breast cancer, prostate cancer, ovarian cancer, bladder cancer, and brain cancer. These anti-cancer effects of etomoxir are not only due to mechanisms within the cancer cells themselves, but also due to a reduction of tumor-associated macrophages in the pro-inflammatory tumor microenvironment.
The University of Colorado filed a patent in 2005 to use a combination of Etomoxir and an inhibitor of glycolysis as an anti-inflammatory and anti-carcinogenic agent, but this patent has since expired. In 2019, the Danish company 2 N Pharma was founded to develop a drug against amyotrophic lateral sclerosis and Parkinson's disease based on similar chemistry to Etomoxir. Numiera Therapeutics, a US based pharmaceutical company, announced in 2025 that they secured orphan drug designation on etomoxir for treating malignant glioma, and that they are planning clinical trials in neuro oncology.
References